MealPro/Idaho State Clinical Study. The Anti-Inflammatory Diet.
MealPro Idaho State University Clinical Study Abstract
The clinical study explores the Anti-Inflammatory Diet for women with Interstitial Cystitis (AID-IC), a low-saturated-fat diet, and its effects on women with interstitial cystitis/bladder pain syndrome (IC/BPS).
Using a randomized crossover design, researchers assessed how the diet impacted symptom severity and quality of life. While the protocol was time-intensive, the barriers were manageable.
Findings suggest that the AID-IC diet may reduce symptoms and enhance the quality of life for women with IC/BPS.
Anti-Inflammatory Diet Clinical Study
1. Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic condition affecting millions of women globally, characterized by pelvic pain, urinary frequency, and urgency. Its cause is unknown, and there is no cure. Chronic inflammation is a hallmark of IC/BPS, with elevated proinflammatory markers like CRP and cytokines observed in patients. Flares vary in frequency and severity, significantly impacting quality of life, including work, social interactions, and mental health.
Diet is recognized as a potential trigger for IC/BPS symptoms, with foods like caffeine, alcohol, citrus, artificial sweeteners, and spicy items commonly reported as problematic. Damage to the bladder urothelial barrier, neural cross-talk, and inflammation from dietary components are potential mechanisms linking diet to symptoms. High consumption of saturated fats and ultra-processed foods is associated with increased inflammation and may worsen symptoms.
The Anti-Inflammatory Diet for Interstitial Cystitis (AID-IC) pilot study explored a diet low in saturated fat and rich in antioxidants as a complementary treatment for IC/BPS. Using the Dietary Inflammatory Index (DII) to measure inflammatory potential, the study aimed to evaluate how dietary changes influence symptom severity and quality of life. Results suggest dietary adjustments may play a role in managing IC/BPS symptoms, providing a foundation for future research and dietary recommendations.
2. Materials and Methods
The AID-IC pilot study aimed to evaluate the impact of a plant-based, low-saturated-fat, anti-inflammatory diet on mitigating symptoms of interstitial cystitis/bladder pain syndrome (IC/BPS). The study used a mixed-methods crossover design based on the ADIRA protocol, spanning 11 months from January to November 2021.
Key Components of the study:
Therapeutic Diet:
Focused on anti-inflammatory foods (e.g., rich in vitamins, carotenoids, and certain spices).
Eliminated inflammatory triggers like citrus, tomatoes, and alcohol.
Included balanced meals with proteins, vegetables, whole grains, low-fat dairy, nuts, and oily fish, avoiding red meat.
Meals were pre-prepared, delivered weekly, and analyzed for nutritional composition.
Control Diet:
Followed standard IC/BPS nutrition counseling with participants responsible for their own meals.
Recruitment and Screening:
Included women aged 18+ with physician-diagnosed IC/BPS.
Excluded those with active medical needs, dietary restrictions, or severe comorbidities.
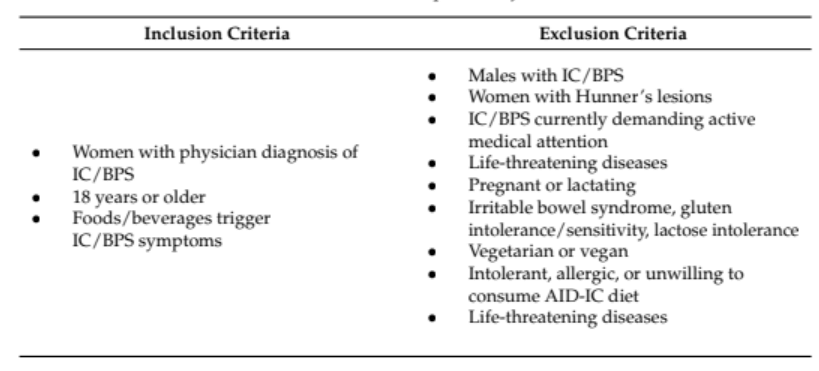
Table 1. Inclusion and exclusion criteria for AID-IC pilot study.
Intervention Structure:
Participants alternated between therapeutic and control diets, with a two-week washout period in between.
Data collection included dietary recalls, quality-of-life surveys, and inflammatory biomarker analysis.
Data Collection:
Primary outcomes: Symptomatology, pain, and quality of life measured using validated tools like GUPI, FSFI, and RICE scales.
Secondary outcomes: Blood tests for inflammatory markers and feedback on diet palatability.
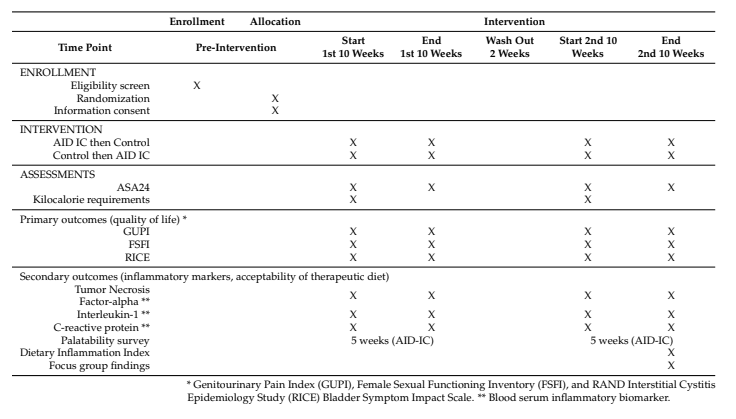
Table 2. Enrollment and data collection stratified by study time.
Qualitative Analysis:
Focus groups gathered insights on adherence, IC/BPS symptoms, quality of life, and overall experience with the diet.
Findings and Analysis:
Statistical methods assessed changes in disease activity, dietary intake, and participant experiences.
A survey evaluated the therapeutic diet’s acceptability and ease of adoption, with feedback informing future protocol adjustments.
This study offers a structured approach to exploring dietary intervention as a complementary IC/BPS treatment, emphasizing its feasibility and potential benefits.
Who made the meals for the clinical study?

MealPro prepared anti-inflammatory meals for this study. Meals are now available for purchase on our website.
The research team contracted with a commercial service for meal production and delivery (MealPro, Sacramento, CA, USA). The anti-inflammatory prepared meals were provided for lunch and dinner on the five weekdays (one-week cycle menu). The portion sizes were pre-established and the meals pre-cooked. Each meal was plated in a vacuum-sealed, microwave/oven-safe container. The packaging allowed for meals to be stacked for cold storage. Two weeks of meals were delivered in thermal boxes on dry ice.
3. Results
The AID-IC pilot study enrolled 37 women over two months to evaluate a plant-based, low-saturated-fat diet’s impact on IC/BPS symptoms. After exclusions for dietary restrictions, protocol demands, and other factors, 18 women were randomized, and 12 participated, with 10 completing the study (83% completion). Participants were predominantly White, non-Hispanic women over 45, with incomes above $50,000 and a high prevalence (70%) of comorbid chronic pain conditions. The most common IC/BPS trigger foods were tomatoes, alcohol, and spicy foods.
Nutrient analysis of the therapeutic diet showed reduced solid and saturated fats, refined grains, and increased seafood and vitamin B12 intake compared to the control diet. The therapeutic diet scored more anti-inflammatory (mean E-DII: -1.50) than the control diet (-0.98), but both were anti-inflammatory overall. A significant decrease in vitamin C intake occurred on the therapeutic diet, though no other macronutrient or micronutrient changes were significant.
Meal acceptability was moderate, with one meatless entrée rated most palatable and salmon dishes rated less favorably. Participants reported the diet as generally easy to follow, with neutral ease for adapting and coping with social situations. No adverse reactions were reported.
4. Discussion
The discussion highlights the limited research on dietary modifications for managing IC/BPS symptoms, with only three prior clinical trials conducted. These studies reported mixed results, with one showing no discrete dietary effects, another finding no significant impact of caffeine on symptoms, and the third showing improvements from systematic food restrictions. The lack of replication underscores the need for further investigation.
The AID-IC pilot study demonstrated the feasibility of using an anti-inflammatory diet for IC/BPS. Participants showed improved disease activity scores (RICE and GUPI) on the therapeutic diet but experienced lower sexual functioning scores, possibly due to the small sample size or confounding factors. The study’s strengths included the use of Registered Dietitian Nutritionists (RDNs) for participant engagement and home-delivered meals to enhance adherence. However, limitations included a small, homogeneous sample, single-site recruitment, delivery logistics, and an insufficiently diverse menu.
Key challenges involved external factors like COVID-19, travel, and weather delays, as well as participant dissatisfaction with meal variety and quality (e.g., “mushy” vegetables and lower-quality frozen salmon). The short two-week washout period may have reduced the observed differences between diet phases, suggesting longer periods could yield more robust findings.
Despite these limitations, the study’s findings support the potential benefits of an anti-inflammatory diet for IC/BPS. It underscores the need for larger, multi-site trials with diverse populations to establish conclusive evidence and improve generalizability. Adjustments to menu planning and logistical procedures could enhance future research outcomes.
5. Conclusion
The AID-IC protocol is a viable approach for studying dietary modifications in IC/BPS patients. The study showed symptom relief and improved quality of life for participants, but larger, more diverse samples are needed to enhance reliability and generalizability. Enhancements in meal design and production could further optimize the effectiveness of an anti-inflammatory diet.
References:
This original cree-reviewed paper of this clinical study was published by Idaho Stat University as: Anti-Inflammatory Diet for Women with Interstitial Cystitis/Bladder Pain Syndrome: The AID-IC Pilot Study and was authored by Barbara Gordon 1, Cynthia Blanton, Rebekah Ramsey, Andrea Jeffery, Laura Richey, and Rachel Hulse.
MealPro was the chosen meal delivery partner for this study and made all the meals for the clinical study. The clinically researched anti-inflammatory meal plan is available for purchase on our website.

Picture of the Peer Reviewed Paper.
Read the whole peer-reviewed paper here
.Department of Nutrition and Dietetics, Idaho State University, 1311 E Central Drive, Meridian, ID 83642, USA; [email protected] (A.J.); [email protected] (L.R.)
Department of Nutrition and Dietetics, Idaho State University, 921 South 8th Avenue, Pocatello, ID 83209, USA; [email protected] (C.B.); [email protected] (R.R.); [email protected] (R.H.)
MealPro: 7433 Greenback Ln, Citrus Heights CA 95610